- 1Department of Science in Korean Medicine, Graduate School, Kyung Hee University, Seoul, South Korea
- 2Department of Preventive Medicine, College of Korean Medicine, Kyung Hee University, Seoul, South Korea
- 3Pharmacological Toxicology Laboratory, Jeil Pharmaceutical, Yongin-si, South Korea
- 4Clinical Medicine Division, Korea Institute of Oriental Medicine, Daejeon, South Korea
- 5Department of Surgery, Beth Israel Deaconess Medical Center and Harvard Medical School Boston, Boston, MA, United States
- 6Department of Korean Medicine, College of Korean Medicine, Graduate School, Kyung Hee University, Seoul, South Korea
Prostate cancer is the second most commonly diagnosed cancer, and prostate cancer is the second most common cause of cancer death in United States men after lung cancer. Many therapies are used to treat prostate cancer, and chemotherapy is one of the most relevant treatments. However, chemotherapy has many side effects, and repeated administration of chemotherapeutic agents leads to acquired resistance. Thus, new drugs with few side effects are needed. We investigated the molecular mechanism of action of JI017 in human prostate cancer cells. We identified an endoplasmic reticulum (ER) stress pathway that depended on the reactive oxygen species (ROS) pathway and played a crucial role in JI017-induced apoptosis. We measured cell viability by the MTS assay to determine the effect of JI017. Analysis of apoptosis, mitochondrial dysfunction, and cell cycle features was performed by flow cytometry. We used western blot and RT-PCR to measure the levels of the proteins of the unfolded protein response (UPR) pathway and apoptosis markers. Immunoprecipitation assay and transfection were used to determine the expression levels of proteins interacting with the pathways influenced by JI017 in prostate cancer cells. The anticancer effects induced by JI017 were evaluated. JI017 induced cell death that regulated apoptotic molecules and caused cell cycle arrest that inhibited the proliferation of cancer cells. Moreover, JI017 generated ROS. Accumulation of ROS caused ER stress through the PERK–eIF2α–CHOP and IRE1α-CHOP pathways. Furthermore, persistent activation of the UPR pathway induced by JI017 treatment triggered mitochondrial dysfunction, including dissipation of mitochondrial membrane potential, which activated intrinsic apoptotic pathway in human prostate cancer cells. The data indicated that N-acetyl-L-cysteine diminished apoptosis. We demonstrated that JI017 induced ER stress and cell death. Anticancer properties of JI017 in prostate cancer cells and in a human prostate cancer model involved ROS-mediated ER stress. Thus, JI017 treatment provides a new strategy for chemotherapy of prostate cancer.
Introduction
Prostate cancer is the second most diagnosed cancer, accounting for the second highest mortality rate in men. Approximately 191,930 new cases and 33,330 deaths were reported in the United States in 2020. Prostate cancer has a high 5 years survival rate due to androgen receptor-targeted treatment (Attard et al., 2011; Siegel et al., 2016). Despite the initial therapeutic effect of androgen therapy, prostate cancer has a poor prognosis, and 30% cases of advanced prostate cancer are drug resistant or have metastasized to other organs, such as the liver and lung, through the lymphatic system (Whitmore Jr, 1984; Carlin and Andriole, 2000). Thus, new treatments are needed for effective elimination of cancer cells by target-specific drugs with few side effects and lack of resistance. Recent studies demonstrated that targeting biochemical alterations in cancer cells may be important for the development of new anticancer strategies (Sen et al., 1992; Burns and Spector, 1994; Hileman et al., 2004).
Recently, interest in herbal medicines, such as antitumor, anticancer, and analgesic drugs, is increasing due to low side effects and high stability (Hedigan, 2010; Majdoub et al., 2019; Kim et al., 2020; Ku et al., 2020). The present study demonstrated the anticancer efficacy of traditional medicine “JI017” consisting of Angelica gigas (Ag), Aconitum carmichaeli (Ac), and Zingiber officinale Roscoe (Zo) at a ratio of 2:1:1 in prostate cancer cells. Previous studies indicated that the components of the herbal extract, including Ag, Ac, and Zo, influences various malignant tumors, such as brain, breast, prostate, colorectal, skin, and pancreatic cancer (Choi et al., 2016; Li et al., 2017; Nedungadi et al., 2019), various inflammatory diseases, and obesity and has neuroprotective effects(Semwal et al., 2015; Zhou et al., 2015; Sowndhararajan and Kim, 2017). Furthermore, extracts from a mixture of Ag, Ac, and Zo have been shown to effective various disease effect (Kim et al., 2016; Ko et al., 2018; Han et al., 2019; Cheon and Ko, 2020; ).
Endoplasmic reticulum is an organelle involved in important cellular functions, including protein folding and secretion (Malhotra and Kaufman, 2007; Feige and Hendershot, 2011). Changes in the environment within ER trigger ER stress by causing the accumulation of misfolded proteins in the ER lumen (Walter and Ron, 2011). The protein overload state of ER is known as “ER stress,” and the induced response is called unfolded protein response; UPR is involved in the treatment of various cancers. Protein overload in the ER lumen is induced by activation of UPR-related sensors: Protein kinase R-like ER kinase (PERK), inositol-requiring enzyme 1 (IRE1), and activating transcription factor 6 (ATF6) (Logue et al., 2013; Rozpedek et al., 2016). These signaling pathways regulate protein translation and restore the folding function of ER. However, persistent ER stress can induce mitochondrial dysfunction and activate apoptotic pathways (Marciniak et al., 2006; Kim et al., 2020).
Reactive oxygen species have recently emerged as important regulators of ER function and UPR activation, and ER stress and increased ROS production were detected on chemotherapy treatment in several malignancies (Cubillos-Ruiz et al., 2017; Lin et al., 2019; Qi et al., 2020; Yang and et al., 2020). ROS are an essential component of the signaling that leads to protein misfolding and ER stress-induced cell death (Verfaillie et al., 2012).
The present study analyzed the activation of the signaling pathways to explain the mechanisms of the effect of JI017 on prostate cancer cells and in a human prostate cancer model. The results indicated that JI017 treatment of prostate cancer cells can trigger pro-apoptotic signaling pathways, including persistent ER stress and mitochondrial dysfunction, by generating ROS.
Materials and Methods
Reagents
Dimethyl sulfoxide was purchased from Sigma-Aldrich (St. Louis, MO, United States). Z-VAD-FMK was provided by R and D. N-Acetylcysteine amide and 4-phenylbutyric acid were provided by Sigma-Aldrich. Dulbecco’s phosphate-buffered saline (DPBS) and Roswell Park Memorial Institute 1640 (RPMI 1640) medium were obtained from WELGENE (Gyeongsan, South Korea).
Preparation of JI017
JI017 consists of Angelica gigas (Ag), Aconitum carmichaeli (Ac), and Zingiber officinale Roscoe (Zo) complexes, JI017 was commissioned to Jaseng Hospital of Korean Medicine (Seoul, South Korea). Briefly, extaracts of Ag, Ac, Zo in 70% ethanol were obtained by boiling for 3 h at 80°C. The ethanol extract was filtered, concentrated using vacuum evaporation and freeze-dried. Dried extract was then dissolved in DMSO to prepare a stock solution of 50 mg/ml. The stock solution was stored at −80°C until used to treat the cells.
HPLC Analysis
HPLC were performed to confirm characteristics of JI017 including each component. JI017 consists of Ag, Ac, Zo complexes, JI017 was commissioned to Hanpoong Pharm and food company (Jeonju, South Korea) The dried roots of Ag, Zo plants were purchased from GM PHARM Co., Ltd (Gyeonggi, South Korea). And the dried root of Ac plant were purchased from Shin Hung PHARM. Co., LTD. (Jeonnam, South Korea). The roots were boiled for 3 h in distilled 70% ethanol. The extract was filtered twice through Whatman grade 2 filter paper (GE Healthcare Life Sciences, Marlborough, MA, United States) to remove insoluble materials. The filtered extract was lyophilized to a powder using a freeze dryer (iLShin biobase, Gyeonggi, South Korea) and stored at 4°C. Characterization of JI017 was based on retention times and UV absorption of standard chemicals at 280 nm. Nodakenin (17 min) and decursin (46 min) were used as the standards for Ag; aconitine (33 min) was used a standard for Ac; and 6-gingerol (36.0 min) was used as a standard for Zo (Figure 1).
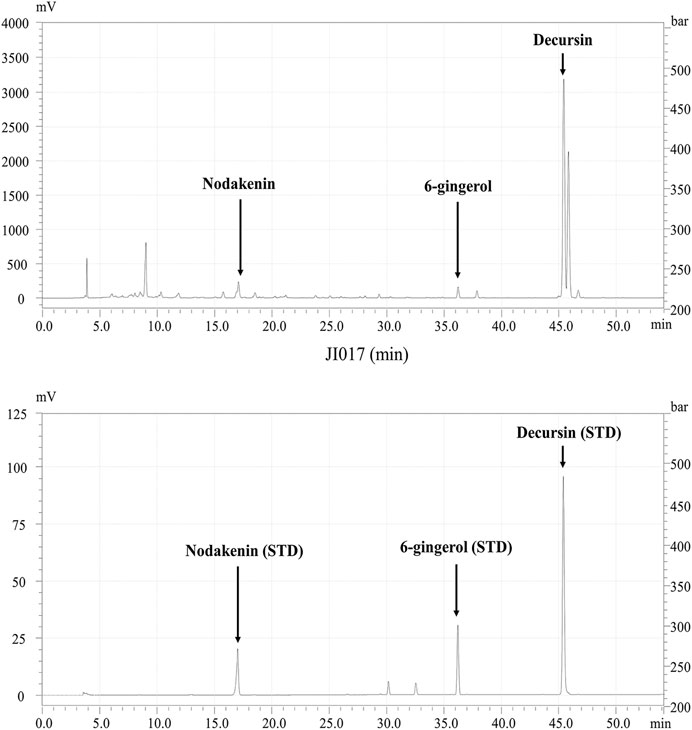
FIGURE 1. HPLC profile of JI017. Identification of the components in JI017; nodakenin, aconitine, 6-gingerol, and decursin were detected at 17, 33, 36, and 46 min, respectively.
Cell Culture
Human prostate cancer cell lines (DU145 and PC3) were purchased from the American Type Culture Collection (ATCC). Cancer cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (JR Scientific) and 1% antibiotic (Invitrogen) at 37°C in a humidified atmosphere containing 5%
Cell Viability Assay
Cell viability was assayed as described previously (Ku et al., 2020) by the MTS assay. Prostate cancer cells were seeded in 96 well plates at a density of 5.0
Colony Formation Assay
Cells were seeded in a 6-well plate at a density of
Western Blot Analysis
Washed cells were harvested, lyzed in cell lysis buffer (50 mM Tris–HCl, pH 7.4; 1% Nonidet P-40; 0.25% sodium deoxycholate; 0.1% sodium dodecyl sulfate; 150 mM NaCl; 1 mM ethylenediaminetetraacetic acid; and a protease inhibitor) on ice for 20 min, and centrifuged at 13,000 rpm (4°C) for 20 min. Quantification of the protein in lyzed cells was performed using a Bradford protein assay kit (Bio-Rad, Hercules, CA, United States). Equivalent amounts of total protein were separated using a 6–15% sodium dodecyl sulfate (SDS)-polyacrylamide gel by electrophoresis and transferred to a nitrocellulose membrane (Protran, Whatman, United Kingdom). The membranes were blocked by incubation for 1 h with 1% skim milk and 1% BSA in PBS-T containing 0.1% Tween 20. The membranes were incubated with primary antibodies. Then, the membranes were incubated with Horse radish peroxidase conjugated secondary IgG antibodies (Calbiochem, San Diego, CA, United States) and probed using an enhanced chemiluminescence detection system (an ECL kit, Amersham Pharmacia Biotech, Inc. Piscataway, NJ, United States).
Antibodies against Bax (sc-7280), Bcl-2 (sc-7382), and β-actin (sc47778) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, United States). Antibodies against cleaved PARP (#9541), cleaved caspase-3 (#9661), cleaved caspase-9 (#7237), cleaved caspase-7 (#9491), GAPDH (#5174s), IRE1 (#3294), p-SAPK/JNK (#4668s), p-ELF2 (#3398), PERK (#5683p), p-PERK (#3179s), C/EBP homologous protein (CHOP) (#2895), and Smac/Diablo (#15108s) were obtained from Cell Signaling Technology (Danvers, MA, United States). Antibodies against GRP78/Bip (ab21685) were obtained from Abcam. Antibodies against cytochrome c (BD556433) were obtained from BD Biosciences (United States).
Cell Cycle Analysis
Flow cytometry was used to analyze the cell cycle and was performed using a BD FACScan system (BD Biosciences, San Jose, CA, United States); the data were analyzed using ModFit LT V2.0 software. Cells were seeded in 60 mm cell culture dishes at a density of
Annexin V-FITC Apoptosis Assay
Flow cytometry was used to analyze apoptosis. Cells were seeded in 60 mm cell culture dishes at a density of
Reactive Oxygen Species (ROS) Assay
Flow cytometry was used to analyze intracellular ROS generation. Cells were seeded in 60 mm cell culture dishes at a density of
Measurement of Mitochondrial Membrane Potential (MMP) by Flow Cytometry
Flow cytometry was used to measure the MMP. Cells were seeded in 60 mm cell culture dishes at a density of
Mitochondrial Fractionation
Mitochondria were isolated from prostate cells using a cell fractionation kit (ab109719) according to the manufacturer’s instructions. Briefly, the cells were seeded in 100 mm cell culture dishes at a density of
RT-PCR
Cells were seeded in 100 mm cell culture dishes at a density of
RT-qPCR
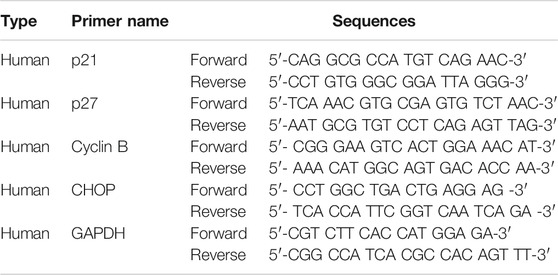
RT-qPCR was performed using an ABL Power SYBR green PCR Master Mix kit (Applied Biosystems) in a 20 μL reaction mixture containing DNA template, 10 pM of the corresponding gene-specific primers, and 2x sensiFAST SYBR Hi-ROX Mix. Specific primers are listed in Table 2.
Immunoprecipitation (IP) Assay
Cells were seeded in 100 mm cell culture dishes at a density of
Transfection
Cells were seeded in 100 mm cell culture dishes at a density of
Statistical Analysis
The results are expressed as the mean ± standard deviation (SD) or mean ± SEM. Statistical significance was determined using one-way analysis of variance followed by Tukey-Kramer multiple comparisons test to evaluate the differences (p < 0.05) between the groups. All experiments were performed at least three times. All statistical analyses were performed using Prism software (GraphPad Software Inc. La Jolla, CA, United States).
Results
JI017 Treatment Induces G2/M Cell Cycle Arrest
We investigated whether JI017 influences the viability of prostate cancer cells. DU145 and PC3 cells were treated with various concentrations of JI017 for 24 h. Then, cell viability was measured by the MTS assay. JI017 significantly suppressed the proliferation of DU145 and PC3 cells in a dose-dependent manner. The IC50 values for these cell lines were 84.10 μg/ml and 118.5 μg/ml, respectively (Figure 2A). Additionally, JI017 treatment significantly reduced colony formation (Figure 2B). The effect of JI017 on the cell cycle of DU145 and PC3 cells was assessed using flow cytometry to determine whether JI017 inhibits cell growth by changing cell cycle progression. The results demonstrated that JI017 induced G2/M phase cell cycle arrest in DU145 and PC3 cells in a dose-dependent manner (0, 40, 80, and 120 μg/ml) (Figure 2C). RT-PCR was used to confirm cell cycle arrest. The results demonstrated that JI017 treatment increased the levels of p21 and p27 and suppressed cyclin B expression in DU145 and PC3 cells (Figure 2D).
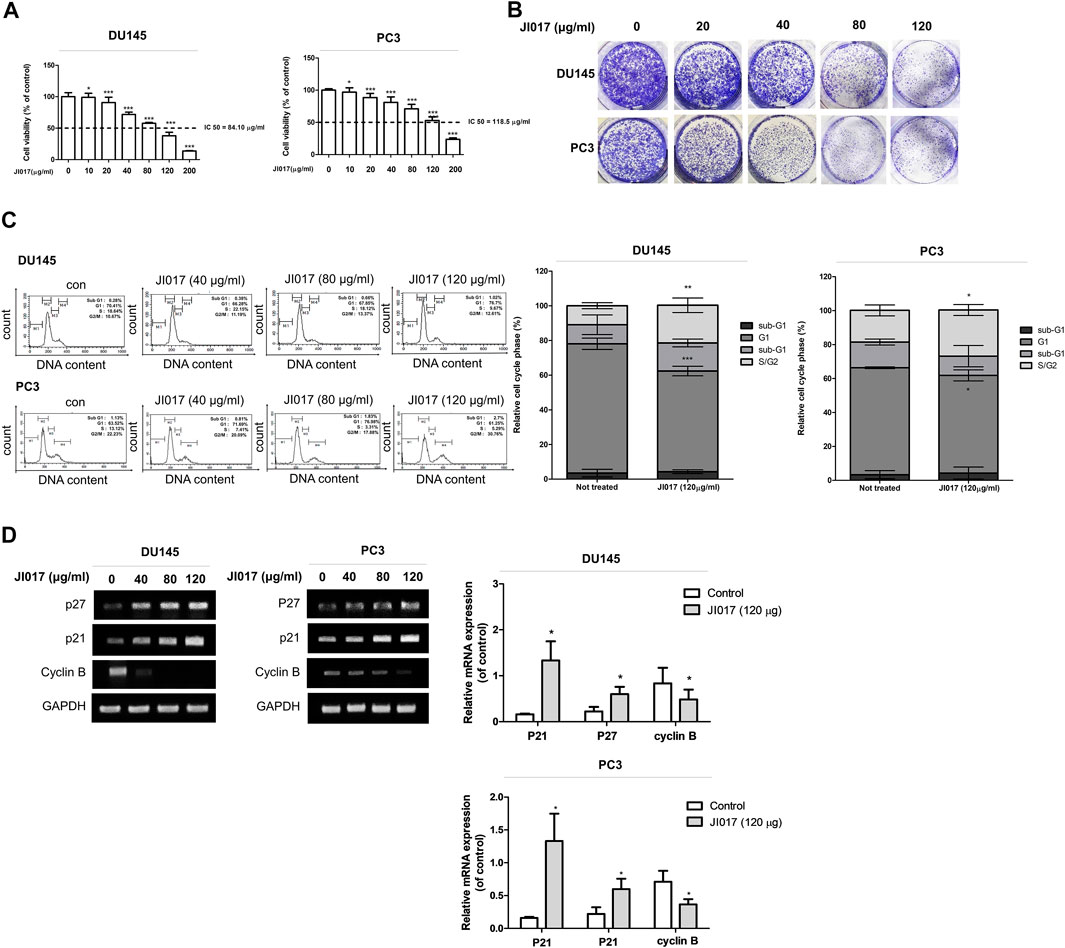
FIGURE 2. The anticancer effect of JI017 treatment induced the G2/M cell cycle arrest and apoptosis of DU145 and PC3 cells. DU145 and PC3 cells were treated with JI017 (40, 80, 120, and 200 μg/ml) for 24 h (A). Cell viability was measured by MTS assay. DU145 and PC3 cells were exposed to JI017 (40, 80, and 120 μg/ml) for 14 days (B). Cell cycle distribution was analyzed using flow cytometry (C). The expression of p21, p27, and cyclin B mRNA was measured by RT-PCR (D). The data are presented as the mean ± SEM. *p < 0.05, **p < 0.01, and ***p < 0.001 compared to untreated cells.
JI017 Treatment Induces Apoptosis of Prostate Cancer Cells
Annexin V-FITC/7-AAD assay was used to determine whether JI017 treatment induces apoptosis of DU145 and PC3 cells. JI017 increased apoptosis in a dose-dependent manner (Figure 3A). The induction of apoptotic pathway in DU145 and PC3 cells by JI017 was confirmed by western blotting. JI017 decreased the levels of Bcl-2 and increased the levels of Bax, cleaved PARP, cleaved caspase-3, and cleaved caspase-9 in DU145 and PC3 cells (Figure 3B). Additionally, we investigated whether JI017 induces cell death by caspase-dependent apoptotic pathway. Pretreatment of the cells with Z-VAD-FMK, a pan caspase inhibitor, before JI017 treatment increased cell viability compared to that of the cells treated with JI017 alone (Figure 3C). Moreover, pretreatment of the cells with Z-VAD-FMK decreased the levels of an apoptosis marker, cleaved PARP, compared to that detected in the cells treated with JI017 alone (Supplementary Figure S1). These results indicated that Bax, Bcl-2, cleaved caspase-3, cleaved caspase-8, and PARP play the critical roles in the effects of JI017 on apoptosis of DU145 and PC3 cells.
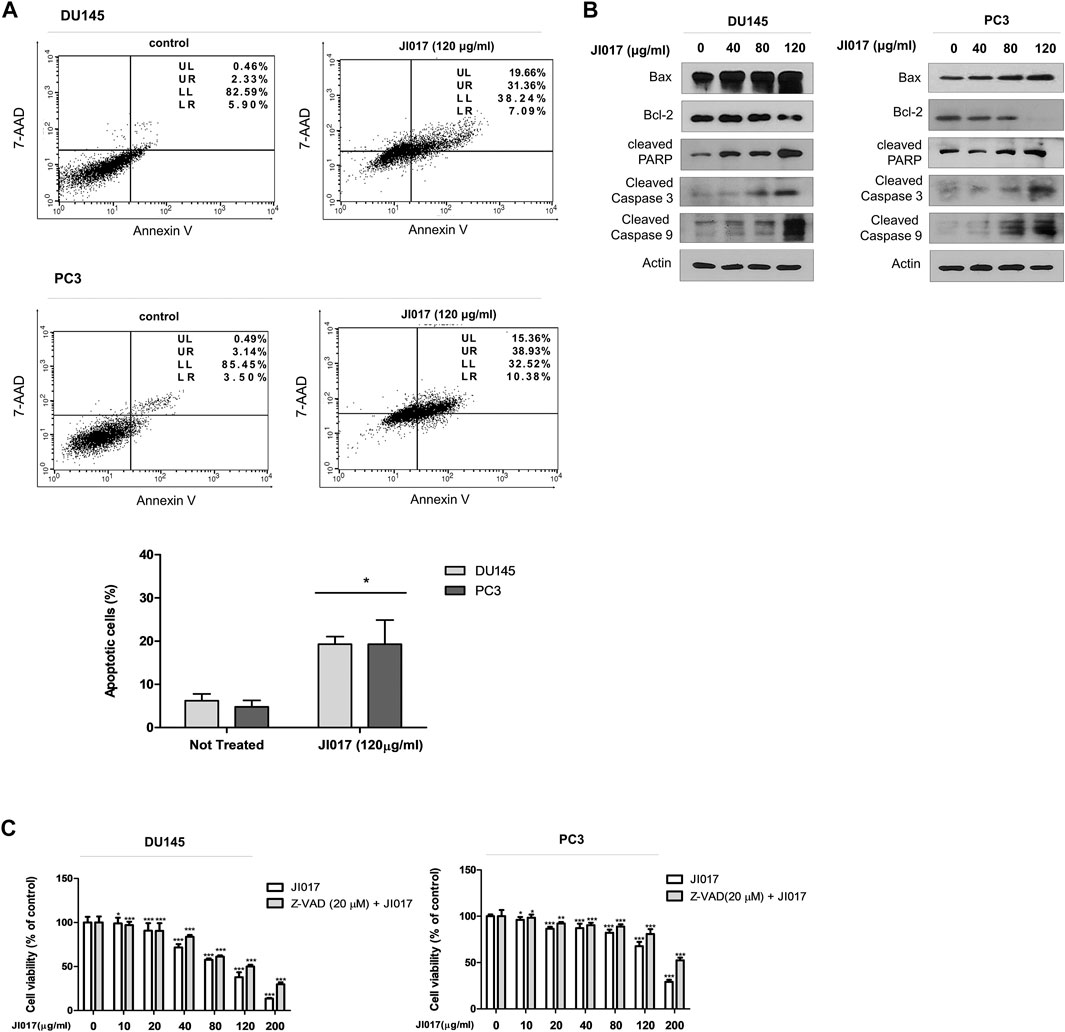
FIGURE 3. DU145 and PC3 cells were treated by indicated drugs for 24 h and used in the Annexin V/7-AAD assay (A). Whole cell lysates were analyzed by western blotting with anti-PARP, anti-cleaved caspase-3 and -9, anti-Bax, anti-Bcl-2, and anti-GAPDH antibodies (B). Pretreatment with Z-VAD-FMK (20 μM) for 1 h followed by treatment with various concentrations of JI017. Cell viability was then measured by MTS assay (C). The data are presented as the mean ± SEM. *p < 0.05, **p < 0.01, and ***p < 0.001 compared to untreated cells.
JI017 Treatment Increases the Generation of Intracellular ROS
ROS are small molecules that play central roles in several signaling pathways(Moloney and Cotter, 2018). Excessive ROS accumulation can induce oxidative damage to lipids, proteins, and DNA in cancer cells and lead to activated signaling pathways that promote apoptosis via extrinsic or intrinsic mechanisms(Engel and Evens, 2006; Srinivas et al., 2019).
We investigated whether JI017 treatment induces intracellular ROS accumulation and measured the level of ROS by using the fluorescent dye 2′,7′-dichlorofluorescein diacetate (DCFH-DA). The cells were treated with various concentrations of JI017 for 24 h and labeled with DCFH-DA, and signal intensity was measured using a flow cytometer. JI017 treatment increased the DCF-dependent signal in a dose-dependent manner (0, 40, 80, 120, and 200 μg/ml) (Figure 4A). Thus, JI017 treatment induced intracellular ROS generation. Then, we investigated whether JI017-induced apoptosis is mediated by ROS generation. Addition of JI017 to the cells pretreated with NAC (10 mM) resulted in a decrease in cell death compared with that detected in cells treated with JI017 alone. Additionally, a decrease in the intensity of the DCF-dependent signal confirmed ROS blockade (Figures 4B,C). Moreover, addition of JI017 to the cells pretreated with NAC decreased the level of an apoptosis marker, cleaved PARP, compared with that in the cells treated with JI017 alone (Supplementary Figure S2). These results suggested that ROS production may be the main factor in the mechanism of apoptosis of prostate cancer cells induced by the treatment with JI017.
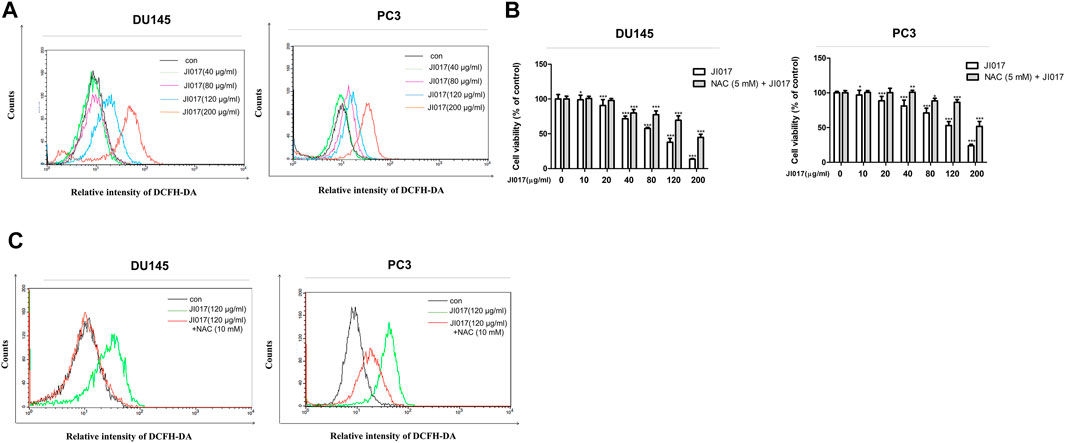
FIGURE 4. JI017 treatment induced ROS accumulation in prostate cancer cells. Intracellular ROS levels in DU145 and PC3 cells treated with JI017 (40, 80, 120, and 200 μg/ml) for 24 h were determined by flow cytometry. Cells were labeled with DCFH-DA (10 μM) for 0.5 h (A). Pretreatment with NAC (10 mM) for 1 h was followed by treatment with various concentrations of JI017. Cell viability was measured using the MTS assay (B). The levels of ROS were measured by flow cytometry (C). The data are presented as the mean ± SEM. *p < 0.05, **p < 0.01, and ***p < 0.001 compared to untreated cells.
Activation of the ER Stress Pathway Depends on ROS Generation
A recent report showed that ROS generation can induce mitochondrial apoptosis via the ER stress pathway (Tabas and Ron, 2011; Kim and Kim, 2018). To confirm the role of the ER stress pathway, we determined whether JI017 influences the UPR signaling, such as protein kinase R-like ER kinase (PERK), inositol-requiring enzyme 1α (IRE1α), and activating transcription factor 6 (ATF6) (Marciniak et al., 2006; Liu et al., 2013). We investigated the UPR pathway by using western blotting. The levels of the components of the ER stress pathway, such as GRP78/BIP, p-PERK, p-ELF2α, p-IRE1α, p-JNK, and CHOP, were significantly increased in a dose-dependent manner (0, 40, 80, and 120 μg/ml JI017) (Figure 5A). CHOP is a master regulator of the initiation of ER stress-induced cell death(Hu et al., 2018). Thus, we investigated whether JI017 treatment significantly enhances the expression of CHOP mRNA by using RT-PCR (Figure 5B, Supplementary Figure S3). The results showed that JI017 treatment induced cell death by activating the UPR pathway. Additional confirmation was obtained using an ER stress inhibitor, 4-PBA (4-phenylbutyric acid); the cells were pretreated with 4-PBA before the treatment with JI017. JI017 treatment of the cells pretreated with 4-PBA (10 mM) resulted in higher cell viability than that of the cells treated with JI017 alone (Figure 5C). These results suggested that ER stress may be the main factor in the mechanism of cell death of prostate cancer cells induced by the treatment with JI017.
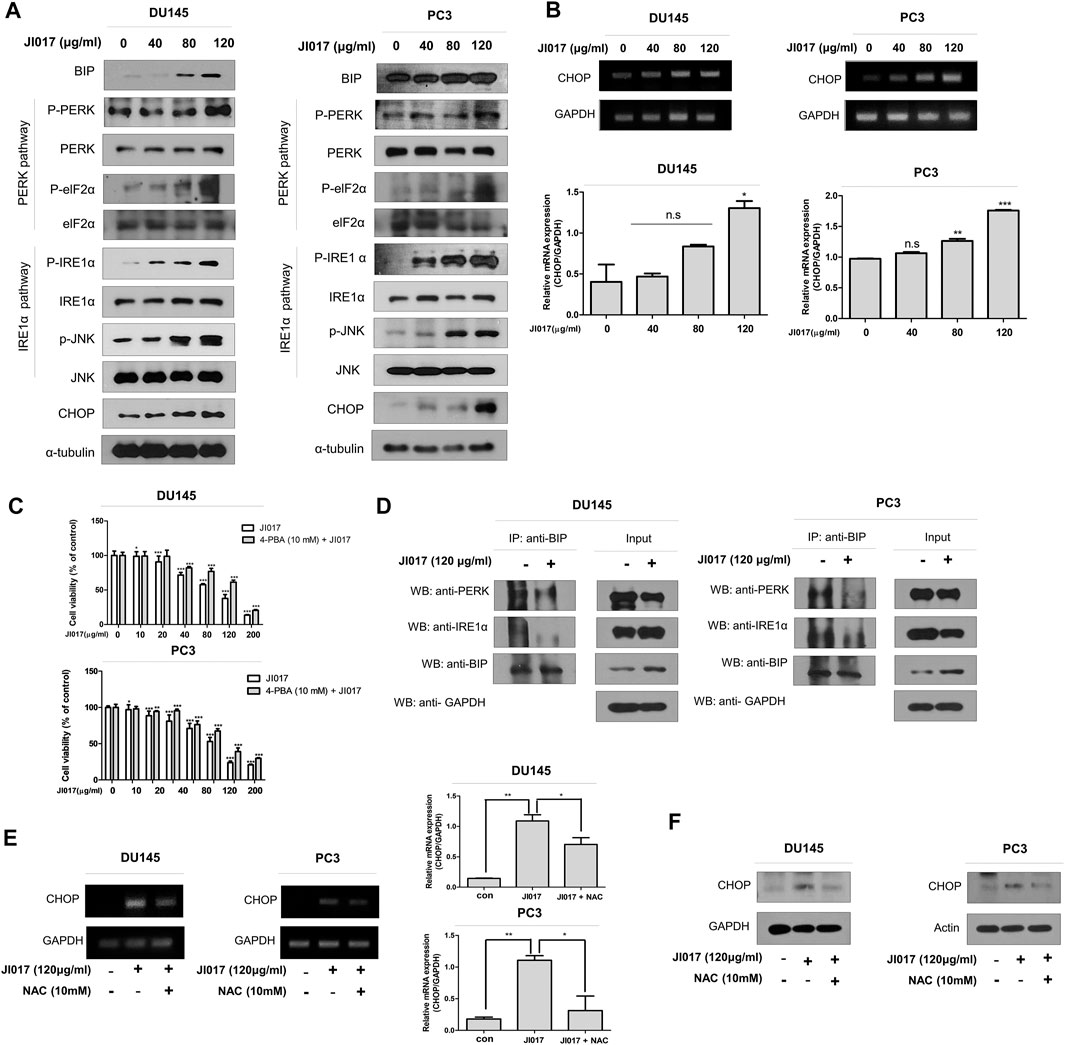
FIGURE 5. JI017 treatment activated UPR signaling in DU145 and PC3 cells. DU145 and PC3 cells were treated with JI017 (40, 80, and 120 μg/ml) for 24 h. Whole cell lysates were analyzed by western blotting with anti-GRP78/BIP, anti-p-PERK, anti-p-ELF2α, anti-p-IRE1 α, anti-p-JNK, anti-CHOP and anti-GAPDH antibodies (A). The expression of CHOP mRNA was measured by RT-PCR (B). Pretreatment with 4-PBA (10 mM) for 1 h was followed by JI017 treatment. Cell viability was measured by the MTS assay (C). DU145 and PC3 cells were treated with JI017 (120 μg/ml) for 24 h. BIP was immunoprecipitated in DU145 and PC3 cells. Then, the immunoprecipitated proteins were detected by western blotting with anti-PERK and anti-IRE1α antibodies (D). Pretreatment with NAC (10 mM) for 1 h was followed by JI017 treatment. Whole cell lysates were analyzed by western blotting with an anti-CHOP antibody (E). The expression of CHOP mRNA was measured by RT-PCR (F). The data are presented as the mean ± SEM. *p < 0.05, **p < 0.01, and ***p < 0.001 compared to untreated cells.
A previous report suggested that the BIP/GRP78 chaperone is a master regulator within ER and is activated upon dissociation of the BIP/GRP78-PERK, and BIP/GRP78-IRE1 complexes (Lewy et al., 2017). Coimunoprecipitation (IP) using antibodies against BIP/GRP78 was performed to investigate the dissociation of the BIP/GRP78-PERK and BIP/GRP78-IRE1α complexes in JI017-treated prostate cancer cells. JI017 treatment increased the level of the BIP/GRP78 chaperone and decreased the dissociation of the BIP/GRP79-PERK and BIP/GRP78-IRE1α complexes (Figure 5D). These results showed that JI017 treatment enhanced UPR signaling via the interaction between BIP/GRP79 and PERK and IRE1α. We investigated whether ROS generation induces ER stress. The cells were pretreated with NAC before adding JI017. The data of western blotting and RT-PCR confirmed that the treatment of NAC-pretreated prostate cells with JI017 enhanced the attenuated level of the ER stress marker CHOP compared with that detected in the cells treated with JI017 alone (Figures 5E,F). These results showed that JI017 treatment regulated cell death via ROS generation that caused ER stress.
ROS Generation Induces Activation of the UPR Pathway and Mitochondrial Dysfunction
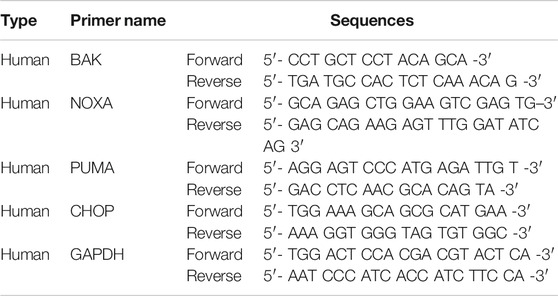
Mitochondrial degradation disrupts cellular homeostasis. Mitochondria mediate apoptosis, a type of cell death that plays a crucial role in cancer. Mitochondria mediate the intrinsic apoptotic pathway (Mayer and Oberbauer, 2003; Wang and Youle, 2009). UPR signaling induces mitochondrial dysfunction through the loss of the mitochondrial membrane potential and increase the production of ROS and mitochondrial oxidation (Cao and Kaufman, 2014). Rhodamine 123 assay was used to determine whether JI017 influences mitochondrial oxidation. JI017 treatment decreased the intensity of the rhodamine 123 signal (Figure 6A). MitoTracker staining was used to determine whether JI017 treatment influences mitochondrial oxidation in the cells. JI017 treatment increased the intensity of the MitoTracker signal (Figure 6B). These results showed that JI017 treatment induced mitochondrial dysfunction via the loss of the MMP (ΔΨm) and mitochondrial oxidation. Additionally, a previous study showed that ER stress mediates apoptosis by inducing proapoptotic Bcl-2 family members, such as PUMA and NOXA; these factors are important for the transfer of death signals from ER to the mitochondria during ER stress. Evaluation of BAK and BH3-only proteins PUMA and NOXA indicated that mitochondrial dysfunction induces the intrinsic apoptotic pathway (Shaltouki et al., 2007). Therefore, we used RT-qPCR to investigate whether JI017 treatment increases the expression of BAK, PUMA, and NOXA mRNAs (Figure 6C). The results showed that the activation of mitochondria-mediated apoptosis regulated the mitochondrial permeability transition pore (MPTP) by activating BH3 proteins and BAK. JI017 treatment resulted in the release of small proteins, such as cytochrome c and Smac/Diablo, to the cytosol via the interaction between BH3-only proteins and BAK; thus, the drug treatment significantly increased the amount of cytochrome c and Smac/Diablo released into the cytosol (Figure 6D). Cells were pretreated with 4-PBA before treatment with JI017 to determine whether the ER stress inhibitor 4-PBA influences mitochondrial apoptosis. Treatment with JI017 and 4-PBA (10 mM) decreased the levels of the markers of intrinsic apoptotic pathway and ER stress (Figure 6E). Thus, JI017 treatment is essential for the induction of mitochondrial apoptotic pathway via ER stress that is dependent on ROS production in prostate cancer cells.
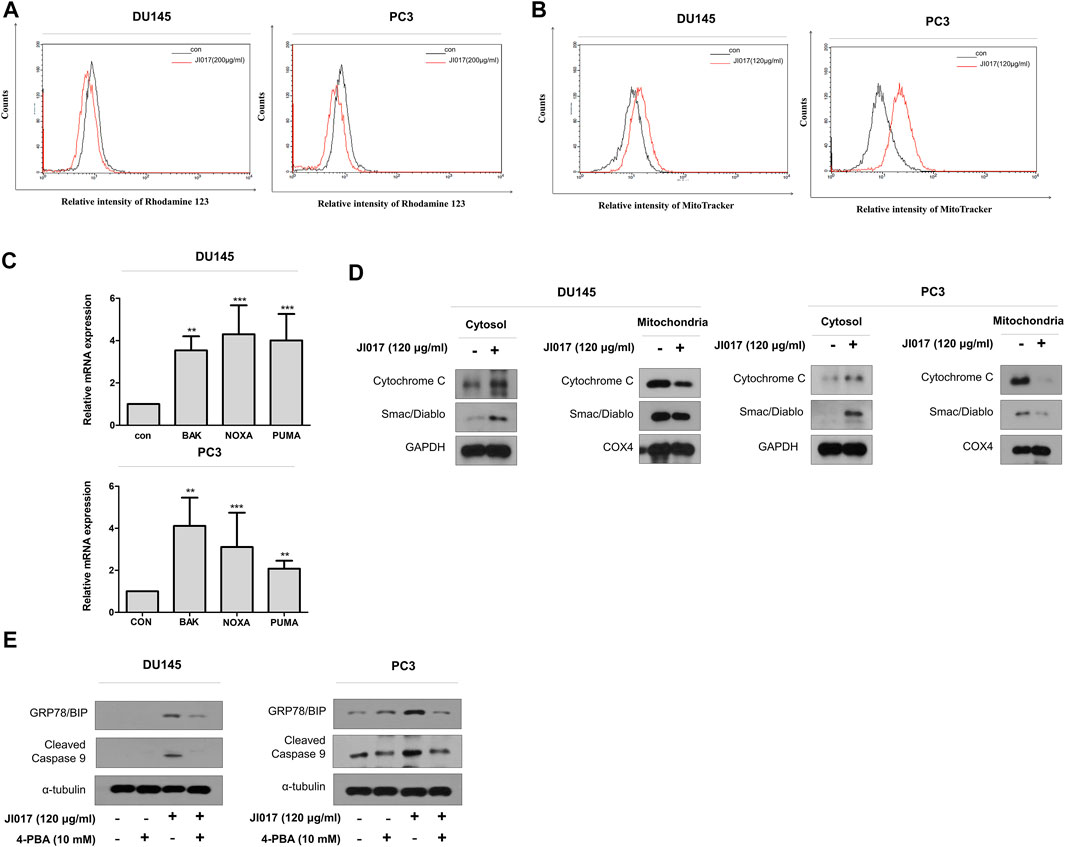
FIGURE 6. Induction of the mitochondrial dysfunction response by UPR signaling. DU145 and PC3 cells were treated with JI017 for 24 h and subjected to the rhodamine 123 assay (A). DU145 and PC3 cells were treated with JI017 for 24 h and stained with MitoTracker (300 nM) (B). The expression of BAK, NOXA, and PUMA mRNAs was measured by RT-qPCR (C). Mitochondrial, and cytosolic extracts of cultured cells were prepared and analyzed by western blotting to detect the levels of cytochrome c and Smac/Diablo (D). Effect of an ER stress inhibitor (4-PBA) on JI017-induced mitochondrial apoptosis pathway. Pretreatment with 4-PBA (10 mM) for 1 h was followed by JI017 treatment. Whole cell lysates were analyzed by western blotting with anti-CHOP, anti-cleaved caspase-9, and anti-GAPDH antibodies (E). The data are presented as the mean ± SEM. *p < 0.05, **p < 0.01, and ***p < 0.001 compared to untreated cells.
The ER Stress Pathway Depends on the ROS Pathway and Plays a Crucial Role in JI017-Induced Apoptosis
Knockdown experiments using CHOP siRNA were performed to determine whether inhibition of ER stress regulates JI017-induced apoptosis. The results showed that CHOP siRNA increased cell viability compared with that detected in the cells treated with JI017 alone. The treatment with JI017 and CHOP siRNA produced greater attenuation of the levels of cleaved caspase-7 and -9 and cleaved PARP than that achieved by treatment with JI017 alone (Figures 7A,B). Overall, our results indicated that JI017 plays an important role in ER stress and induces mitochondrial pathway of apoptosis by activating UPR signaling via ROS generation in prostate cancer cells (Figure 7C).
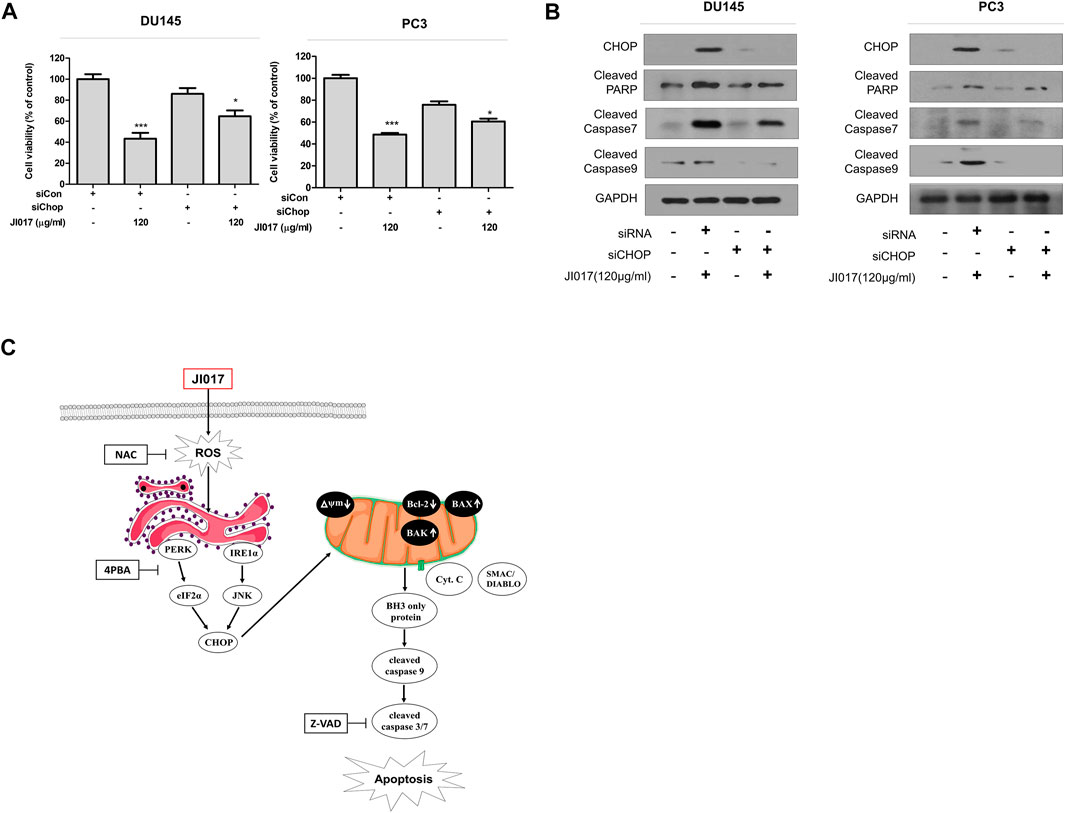
FIGURE 7. Knockdown of CHOP signaling blocks apoptosis induced by JI017 treatment of prostate cell lines. DU145 and PC3 cells were transfected with CHOP siRNA and treated with JI017. Cell viability was measured by the MTS assay (A). Whole cell lysates were analyzed by western blotting with anti-CHOP, anti-cleaved caspase-3, anti-cleaved caspase-7, anti-cleaved caspase-9, anti-CHOP, and anti-GAPDH antibodies (B). Schematic representation of JI017 treatment of prostate cancer cells (C). The data are presented as the mean ± SEM. *p < 0.05, **p < 0.01, and ***p < 0.001 compared to untreated cells.
Discussion
Prostate cancer is the second most common and highly deadly malignancy in men. An increase in the incidence rate of prostate cancer has been observed in Asia and Europe (Siegel et al., 2016). An increase in the incidence rate of prostate cancer, severe side effects, and acquired resistance require identification of effective anticancer drugs. The methods of cancer treatment depend on the stage of prostate cancer development. Therefore, treatments for each stage are needed. Current treatment of prostate cancer is divided into four major stages; each stage includes surgery to remove the prostate gland, chemotherapy, hormone therapy, radical prostatectomy, and radiation. Briefly, Localized prostate cancer, called stage 1–2, is cancer that has not spread to lymph nodes or distant organs. There are three treatments for localized prostate cancer (stage 1–2 cancer), including surgery and radiation therapy, surveillance (Caubet et al., 1997; Teo et al., 2019). And advanced prostate cancer, called Stage 3–4, is that prostate cancer spreads beyond the prostate gland to other parts of the body, such as the lymph nodes or bone, chemotherapy is usually used to treated advanced prostate cancer (stage 3–4) (Knudsen and Vasioukhin, 2010; Berruti et al., 2011). Chemotherapy is effective because it kills cancer that has spread throughout the body. However, chemotherapy has many side effects such as temporary hair loss, and repeated administration leads to acquired resistance and multiple genetic alteration (Yagoda and Petrylak, 1993; Bogdanos et al., 2003; Fizazi et al., 2007). Thus, many novel agents derived from natural medical herbs and herbal medicine mixtures associated with potent anticancer effects have been identified in recent decades, and JI017 is one of these promising agent.
The present study investigated the anticancer effects of JI017 treatment on prostate cancer cells mediated by the signaling pathways. JI017 treatment induced the G2/M phase arrest by increasing the expression of p21 and p27 and the inactivation of cyclin B. Cell cycle arrest is known to be the main mechanism controlling the proliferation of cancer cells and cancer progression (Vermeulen et al., 2003). Thus, JI017 treatment suppressed the growth of prostate cancer cells. Additionally, the induction of cancer cell death by promoting apoptosis has been proposed as an effective therapeutic approach for elimination of cancer cells. Apoptotic cell death is triggered by biochemical functions, such as intracellular ROS generation. Excessive levels of ROS can damage DNA, lipids, and proteins by regulating various pathways, including ER stress, migration, and autophagy (Lin et al., 2019). The results of the present study indicated that an increase in ROS induced by JI017 treatment in prostate cells induced ER stress.
ER stress is an apoptotic signaling pathway involved in several diseases. Previous studies demonstrated that ER stress, known as a “double-edged sword,” plays a dual role in cell survival and death. ER stress can induce apoptosis under stress conditions and survival of cancer cells under adverse conditions. The entry of unfolded proteins into ER activates the UPR pathway. Glucose-regulated protein 78 (GRP78) is a critical regulating sensor protein of the inositol-requiring enzyme 1α (IRE1α) and protein kinase R (PKR)-like kinase (PERK) sensors in various UPR pathways. GPR78/BIP is an ER chaperone induced during ER stress and is critical for the formation of disulfide bonds in proteins. ER stress response sensors are inactive under physiological conditions due to GRP78/BIP binding. However, stress conditions induce dissociation of GRP78/BIP from the sensors and subsequent activation of the sensor proteins, which leads to the activation of eukaryotic translation initiation factor-2α (eIF2α); moreover, IRE1α phosphorylates c-Jun N-terminal protein kinase-1 (JNK1). JNK signaling and UPR sensors activate CHOP. Then, persistent ER stress can trigger the apoptotic pathway (Kleizen and Braakman, 2004; Fagone and Jackowski, 2009; Rozpedek et al., 2016).
Furthermore, the induction of apoptosis by ER stress has been shown to be mediated by the mitochondrial pathway (Shen et al., 2001; Pandey et al., 2006). This pathway involves Bcl‐2 family members and pro-apoptotic Bax and BH3 proteins and is mediated by opening of mitochondrial outer membrane pores. Upon mitochondrial degradation, the changes in apoptotic factors induce the loss of mitochondrial membrane potential (ΔΨm), and small molecules, such as cytochrome c and Smac/Diablo, are subsequently released through the mitochondrial pores, activating the mitochondrial apoptotic pathway via cleaved caspase-3 and -9 to induce apoptosis (McCullough et al., 2001; Kuwana et al., 2005; Maes et al., 2017).
The results of the present study indicated that JI017 treatment induced apoptosis through the activation of cleaved caspase-3 and cleaved caspase-9 in prostate cancer cells. Previous studies have suggested that ER provides an important connection between ROS generation and mitochondrial apoptosis through activation of the UPR pathway. Intracellular ROS production leads to the activation of the UPR pathways, such as the PERK-ELF2a-CHOP and IRE1a-JNK-CHOP pathways, that promote ER stress. The results of the present study indicated that JI017 treatment upregulated CHOP and induced cell death in prostate cancer cells. Interestingly, cell death induced by JI017 treatment can be regulated by the ROS scavenger NAC. The results showed that the JI017 treatment-induced UPR pathway was abolished by ROS scavengers, and we speculate that ROS-activated UPR signaling may be involved in the anticancer activity of JI017. Additionally, we observed the loss of MMP and mitochondrial oxidation. JI017 treatment induced the release of mitochondrial cytochrome c and Smac/Diablo through the mitochondrial pores formed due to the loss of MMP. Activation of UPR signaling can induce the mitochondrial apoptosis pathway. The results of the present study indicated that mitochondrial apoptosis was inhibited by 4-PBA (an ER stress inhibitor) and siCHOP based on the recovery of mitochondrial cell death markers, such as cleaved caspase-9. Thus, the findings of the present study suggested that ER stress may be involved in apoptosis induced by mitochondrial dysfunction. However, the mechanism of apoptotic signal transmission from the mitochondria to the ER in JI017-treated cells is unclear. Further research in this direction is needed to understand the mechanisms of these effects.
Conclusion
The role of the changes in ER molecular pathways in prostate cancer cells in response to ROS generation was investigated, and JI017 was identified as a promising agent that kills cancer cells. Therefore, JI017 is suggested as a novel therapeutic strategy to improve the treatment of prostate cancer.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
MJK carried out the experiments and drafted the manuscript. JMK, SHH, HIK, YYK, and SYC revised the research and manuscript and assisted in the research. JSP performed experiments and data analysis. DHJ contributed to the design of this study and performed data analysis. JMK and YCS guided the research and revised and submitted the manuscript. SGK supervised the experiments and corrected the manuscript. All the authors read and approved the final manuscript.
Funding
This work was supported by the national Research Foundation of Korea (NRF) grant funded by Korean government (MIST) (no. 2020R1A5A2019413).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank our colleagues from the Laboratory of Prevention Medicine for technical assistance and useful discussions.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.683575/full#supplementary-material
References
Attard, G., Richards, J., and de Bono, J. S. (2011). New Strategies in Metastatic Prostate Cancer: Targeting the Androgen Receptor Signaling Pathway. Clin. Cancer Res. 17, 1649–1657. doi:10.1158/1078-0432.ccr-10-0567
Berruti, A., Pia, A., and Terzolo, M. (2011). Abiraterone and Increased Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 365, 766–768. doi:10.1056/NEJMc1107198
Bogdanos, J., Karamanolakis, D., Tenta, R., Tsintavis, A., Milathianakis, C., Mitsiades, C., et al. (2003). Endocrine/paracrine/autocrine Survival Factor Activity of Bone Microenvironment Participates in the Development of Androgen Ablation and Chemotherapy Refractoriness of Prostate Cancer Metastasis in Skeleton. Endocr. Relat. Cancer 10, 279–289. doi:10.1677/erc.0.0100279
Burns, C. P., and Spector, A. A. (1994). Biochemical Effects of Lipids on Cancer Therapy. J. Nutr. Biochem. 5, 114–123. doi:10.1016/0955-2863(94)90082-5
Cao, S. S., and Kaufman, R. J. (2014). Endoplasmic Reticulum Stress and Oxidative Stress in Cell Fate Decision and Human Disease. Antioxid. Redox Signal. 21, 396–413. doi:10.1089/ars.2014.5851
Carlin, B. I., and Andriole, G. L. (2000). The Natural History, Skeletal Complications, and Management of Bone Metastases in Patients with Prostate Carcinoma. Cancer 88, 2989–2994. doi:10.1002/1097-0142(20000615)88:12+<2989::aid-cncr14>3.0.co;2-q
Caubet, J.-F., Tosteson, T. D., Dong, E. W., Naylon, E. M., Whiting, G. W., Ernstoff, M. S., et al. (1997). Maximum Androgen Blockade in Advanced Prostate Cancer: a Meta-Analysis of Published Randomized Controlled Trials Using Nonsteroidal Antiandrogens. Urology 49, 71–78. doi:10.1016/s0090-4295(96)00325-1
Cheon, C., and Ko, S.-G. (2020). Phase I Study to Evaluate the Maximum Tolerated Dose of the Combination of SH003 and Docetaxel in Patients with Solid Cancer. Medicine (Baltimore) 99, e22228. doi:10.1097/md.0000000000022228
Choi, H. S., Cho, S.-G., Kim, M. K., Kim, M. S., Moon, S. H., Kim, I. H., et al. (2016). Decursin in Angelica gigas Nakai (AGN) Enhances Doxorubicin Chemosensitivity in NCI/ADR-RES Ovarian Cancer Cells via Inhibition of P-Glycoprotein Expression. Phytother. Res. 30, 2020–2026. doi:10.1002/ptr.5708
Cubillos-Ruiz, J. R., Bettigole, S. E., and Glimcher, L. H. (2017). Tumorigenic and Immunosuppressive Effects of Endoplasmic Reticulum Stress in Cancer. Cell 168, 692–706. doi:10.1016/j.cell.2016.12.004
Engel, R. H., and Evens, A. M. (2006). Oxidative Stress and Apoptosis: a New Treatment Paradigm in Cancer. Front. Biosci. 11, 300–312. doi:10.2741/1798
Fagone, P., and Jackowski, S. (2009). Membrane Phospholipid Synthesis and Endoplasmic Reticulum Function. J. Lipid Res. 50, S311–S316. doi:10.1194/jlr.r800049-jlr200
Feige, M. J., and Hendershot, L. M. (2011). Disulfide Bonds in ER Protein Folding and Homeostasis. Curr. Opin. Cell Biol. 23, 167–175. doi:10.1016/j.ceb.2010.10.012
Fizazi, K., Le Maitre, A., Hudes, G., Berry, W. R., Kelly, W. K., Eymard, J.-C., et al. (2007). Addition of Estramustine to Chemotherapy and Survival of Patients with Castration-Refractory Prostate Cancer: a Meta-Analysis of Individual Patient Data. Lancet Oncol. 8, 994–1000. doi:10.1016/s1470-2045(07)70284-x
Han, B. H., Yoon, J. J., Kim, H. Y., Ahn, Y. M., Jin, S. N., Wen, J. F., et al. (2019). Inhibitory Effects of Herbal Decoction Ojeoksan on Proliferation and Migration in Vascular Smooth Muscle Cells. J. Physiol. Pharmacol. 70. doi:10.26402/jpp.2019.2.12
Hedigan, K. (2010). Herbal Medicine Reduces Chemotherapy Toxicity. Nat. Rev. Drug Discov. 9, 765. doi:10.1038/nrd3280
Hileman, E. O., Liu, J., Albitar, M., Keating, M. J., and Huang, P. (2004). Intrinsic Oxidative Stress in Cancer Cells: a Biochemical Basis for Therapeutic Selectivity. Cancer Chemother. Pharmacol. 53, 209–219. doi:10.1007/s00280-003-0726-5
Hu, H., Tian, M., Ding, C., and Yu, S. (2018). The C/EBP Homologous Protein (CHOP) Transcription Factor Functions in Endoplasmic Reticulum Stress-Induced Apoptosis and Microbial Infection. Front. Immunol. 9, 3083. doi:10.3389/fimmu.2018.03083
Kim, C., and Kim, B. (2018). Anti-Cancer Natural Products and Their Bioactive Compounds Inducing ER Stress-Mediated Apoptosis: A Review. Nutrients 10. doi:10.3390/nu10081021
Kim, T. W., Cheon, C., and Ko, S. G. (2020). SH003 Activates Autophagic Cell Death by Activating ATF4 and Inhibiting G9a under Hypoxia in Gastric Cancer Cells. Cell Death Dis 11, 717. doi:10.1038/s41419-020-02924-w
Kim, T. W., Hong, D.-W., Kang, C.-M., and Hong, S. H. (2020). A Novel PPARɣ Ligand, PPZ023, Overcomes Radioresistance via ER Stress and Cell Death in Human Non-small-cell Lung Cancer Cells. Exp. Mol. Med. 52, 1730–1743. doi:10.1038/s12276-020-00511-9
Kim, W., Park, S., Choi, C., Kim, Y. R., Park, I., Seo, C., et al. (2016). Evaluation of Anti-inflammatory Potential of the New Ganghwaljetongyeum on Adjuvant-Induced Inflammatory Arthritis in Rats. Evid. Based Complement. Alternat Med. 2016, 1230294. doi:10.1155/2016/1230294
Kleizen, B., and Braakman, I. (2004). Protein Folding and Quality Control in the Endoplasmic Reticulum. Curr. Opin. Cell Biol. 16, 343–349. doi:10.1016/j.ceb.2004.06.012
Knudsen, B. S., and Vasioukhin, V. (2010). Mechanisms of Prostate Cancer Initiation and Progression. Adv. Cancer Res. 109, 1–50. doi:10.1016/b978-0-12-380890-5.00001-6
Ko, Y., Go, H. Y., Han, I. S., Lee, K. Y., Kim, T. H., Lee, J. M., et al. (2018). Efficacy and Safety of Ojeok-San in Korean Female Patients with Cold Hypersensitivity in the Hands and Feet: Study Protocol for a Randomized, Double-Blinded, Placebo-Controlled, Multicenter Pilot Study. Trials 19, 662. doi:10.1186/s13063-018-3013-9
Ku, J. M., Hong, S. H., Kim, H. I., Kim, M. J., Kim, S. K., Kim, M., et al. (2020). Synergistic Anticancer Effect of Combined Use of Trichosanthes Kirilowii with Cisplatin and Pemetrexed Enhances Apoptosis of H1299 Non-small-cell Lung Cancer Cells via Modulation of ErbB3. Phytomedicine 66, 153109. doi:10.1016/j.phymed.2019.153109
Kuwana, T., Bouchier-Hayes, L., Chipuk, J. E., Bonzon, C., Sullivan, B. A., Green, D. R., et al. (2005). BH3 Domains of BH3-Only Proteins Differentially Regulate Bax-Mediated Mitochondrial Membrane Permeabilization Both Directly and Indirectly. Mol. Cell 17, 525–535. doi:10.1016/j.molcel.2005.02.003
Lewy, T. G., Grabowski, J. M., and Bloom, M. E. (2017). BiP: Master Regulator of the Unfolded Protein Response and Crucial Factor in Flavivirus Biology. Yale J. Biol. Med. 90, 291–300.
Li, J.-j., Chen, W.-l., Wang, J.-y., Hu, Q.-w., Sun, Z.-p., Zhang, S., et al. (2017). Wenshen Zhuanggu Formula Effectively Suppresses Breast Cancer Bone Metastases in a Mouse Xenograft Model. Acta Pharmacol. Sin 38, 1369–1380. doi:10.1038/aps.2017.13
Lin, Y., Jiang, M., Chen, W., Zhao, T., and Wei, Y. (2019). Cancer and ER Stress: Mutual Crosstalk between Autophagy, Oxidative Stress and Inflammatory Response. Biomed. Pharmacother. 118, 109249. doi:10.1016/j.biopha.2019.109249
Lin, Y. N., Jiang, M., Chen, W. J., Zhao, T. J., and Wei, Y. F. (2019). Cancer and ER Stress: Mutual Crosstalk between Autophagy, Oxidative Stress and Inflammatory Response. Biomed. Pharmacother. 118. doi:10.1016/j.biopha.2019.109249
Liu, Z.-W., Zhu, H.-T., Chen, K.-L., Dong, X., Wei, J., Qiu, C., et al. (2013). Protein Kinase RNA- like Endoplasmic Reticulum Kinase (PERK) Signaling Pathway Plays a Major Role in Reactive Oxygen Species (ROS)- Mediated Endoplasmic Reticulum Stress- Induced Apoptosis in Diabetic Cardiomyopathy. Cardiovasc. Diabetol. 12, 158. doi:10.1186/1475-2840-12-158
Logue, S. E., Cleary, P., Saveljeva, S., and Samali, A. (2013). New Directions in ER Stress-Induced Cell Death. Apoptosis 18, 537–546. doi:10.1007/s10495-013-0818-6
Maes, M. E., Schlamp, C. L., and Nickells, R. W. (2017). BAX to Basics: How the BCL2 Gene Family Controls the Death of Retinal Ganglion Cells. Prog. Retin. Eye Res. 57, 1–25. doi:10.1016/j.preteyeres.2017.01.002
Majdoub, Y. O. E., Diouri, M., Arena, P., Arigò, A., Cacciola, F., Rigano, F., et al. (2019). Evaluation of the Availability of Delphinidin and Cyanidin-3-O-Sambubioside from Hibiscus sabdariffa and 6-gingerol from Zingiber Officinale in Colon Using Liquid Chromatography and Mass Spectrometry Detection. Eur. Food Res. Technol. 245, 2425–2433. doi:10.1007/s00217-019-03358-1
Malhotra, J. D., and Kaufman, R. J. (2007). The Endoplasmic Reticulum and the Unfolded Protein Response. Semin. Cell Dev. Biol. 18, 716–731. doi:10.1016/j.semcdb.2007.09.003
Marciniak, S. J., Garcia-Bonilla, L., Hu, J., Harding, H. P., and Ron, D. (2006). Activation-dependent Substrate Recruitment by the Eukaryotic Translation Initiation Factor 2 Kinase PERK. J. Cell Biol 172, 201–209. doi:10.1083/jcb.200508099
Mayer, B., and Oberbauer, R. (2003). Mitochondrial Regulation of Apoptosis. Physiology 18, 89–94. doi:10.1152/nips.01433.2002
McCullough, K. D., Martindale, J. L., Klotz, L.-O., Aw, T.-Y., and Holbrook, N. J. (2001). Gadd153 Sensitizes Cells to Endoplasmic Reticulum Stress by Down-Regulating Bcl2 and Perturbing the Cellular Redox State. Mol. Cell. Biol. 21, 1249–1259. doi:10.1128/mcb.21.4.1249-1259.2001
Moloney, J. N., and Cotter, T. G. (2018). ROS Signalling in the Biology of Cancer. Semin. Cell Dev. Biol. 80, 50–64. doi:10.1016/j.semcdb.2017.05.023
Nedungadi, D., Binoy, A., Vinod, V., Vanuopadath, M., Nair, S. S., Nair, B. G., et al. (2019). Ginger Extract Activates Caspase Independent Paraptosis in Cancer Cells via ER Stress, Mitochondrial Dysfunction, AIF Translocation and DNA Damage. Nutr. Cancer 73, 1–13. doi:10.1080/01635581.2019.1685113
Pandey, B. N., Gordon, D. M., De Toledo, S. M., Pain, D., and Azzam, E. I. (2006). Normal Human Fibroblasts Exposed to High- or Low-Dose Ionizing Radiation: Differential Effects on Mitochondrial Protein Import and Membrane Potential. Antioxid. Redox Signal. 8, 1253–1261. doi:10.1089/ars.2006.8.1253
Qi, J., Zhou, N., Li, L., Mo, S., Zhou, Y., Deng, Y., et al. (2020). Ciclopirox Activates PERK-dependent Endoplasmic Reticulum Stress to Drive Cell Death in Colorectal Cancer. Cell Death Dis 11, 582. doi:10.1038/s41419-020-02868-1
Rozpedek, W., Pytel, D., Mucha, B., Leszczynska, H., Diehl, J. A., and Majsterek, I. (2016). The Role of the PERK/eIF2α/ATF4/CHOP Signaling Pathway in Tumor Progression during Endoplasmic Reticulum Stress. Curr Mol Med 16, 533–544. doi:10.2174/1566524016666160523143937
Semwal, R. B., Semwal, D. K., Combrinck, S., and Viljoen, A. M. (2015). Gingerols and Shogaols: Important Nutraceutical Principles from Ginger. Phytochemistry 117, 554–568. doi:10.1016/j.phytochem.2015.07.012
Sen, S., D’Incalci, M., and Apoptosis, M. (1992). Apoptosis Biochemical Events and Relevance to Cancer Chemotherapy. FEBS Lett. 307, 122–127. doi:10.1016/0014-5793(92)80914-3
Shaltouki, A., Freer, M., Mei, Y., and Weyman, C. M. (2007). Increased Expression of the Pro-apoptotic Bcl2 Family Member PUMA Is Required for Mitochondrial Release of Cytochrome C and the Apoptosis Associated with Skeletal Myoblast Differentiation. Apoptosis 12, 2143–2154. doi:10.1007/s10495-007-0135-z
Shen, X., Ellis, R. E., Lee, K., Liu, C.-Y., Yang, K., Solomon, A., et al. (2001). Complementary Signaling Pathways Regulate the Unfolded Protein Response and Are Required for C. elegans Development. Cell 107, 893–903. doi:10.1016/s0092-8674(01)00612-2
Siegel, R. L., Miller, K. D., and Jemal, A. (2016). Cancer Statistics, 2016. CA: A Cancer J. Clin. 66, 7–30. doi:10.3322/caac.213322016
Sowndhararajan, K., and Kim, S. (2017). Neuroprotective and Cognitive Enhancement Potentials of Angelica gigas Nakai Root: A Review. Sci. Pharm. 85, 21. doi:10.3390/scipharm85020021
Srinivas, U. S., Tan, B. W. Q., Vellayappan, B. A., and Jeyasekharan, A. D. (2019). ROS and the DNA Damage Response in Cancer. Redox Biol. 25, 101084. doi:10.1016/j.redox.2018.101084
Tabas, I., and Ron, D. (2011). Integrating the Mechanisms of Apoptosis Induced by Endoplasmic Reticulum Stress. Nat. Cell Biol 13, 184–190. doi:10.1038/ncb0311-184
Teo, M. Y., Rathkopf, D. E., and Kantoff, P. (2019). Treatment of Advanced Prostate Cancer. Annu. Rev. Med. 70, 479–499. doi:10.1146/annurev-med-051517-011947
Verfaillie, T., Rubio, N., Garg, A. D., Bultynck, G., Rizzuto, R., Decuypere, J.-P., et al. (2012). PERK Is Required at the ER-Mitochondrial Contact Sites to Convey Apoptosis after ROS-Based ER Stress. Cell Death Differ 19, 1880–1891. doi:10.1038/cdd.2012.74
Vermeulen, K., Van Bockstaele, D. R., and Berneman, Z. N. (2003). The Cell Cycle: a Review of Regulation, Deregulation and Therapeutic Targets in Cancer. Cell Prolif 36, 131–149. doi:10.1046/j.1365-2184.2003.00266.x
Walter, P., and Ron, D. (2011). The Unfolded Protein Response: from Stress Pathway to Homeostatic Regulation. Science 334, 1081–1086. doi:10.1126/science.1209038
Wang, C., and Youle, R. J. (2009). The Role of Mitochondria in Apoptosis. Annu. Rev. Genet. 43, 95–118. doi:10.1146/annurev-genet-102108-134850
Whitmore, W. F. (1984). Natural History and Staging of Prostate Cancer. Urol. Clin. North America 11, 205–220. doi:10.1016/s0094-0143(21)00182-8
Yagoda, A., and Petrylak, D. (1993). Cytotoxic Chemotherapy for Advanced Hormone-Resistant Prostate Cancer. Cancer 71, 1098–1109. doi:10.1002/1097-0142(19930201)71:3+<1098::aid-cncr2820711432>3.0.co;2-g
Yang, L., et al. (2020). Reactive Oxygen Species Mediate Anlotinib-Induced Apoptosis via Activation of Endoplasmic Reticulum Stress in Pancreatic Cancer. Cell Death Dis 11, 766. doi:10.1038/s41419-020-02938-4
Keywords: cancer, JI017, er stress, CHOP, ROS, mitochondria cell death
Citation: Kim MJ, Ku JM, Hong SH, Kim HI, Kwon YY, Park J-S, Jung DH, Shin YC and Ko S-G (2021) In vitro Anticancer Effects of JI017 on Two Prostate Cancer Cell Lines Involve Endoplasmic Reticulum Stress Mediated by Elevated Levels of Reactive Oxygen Species. Front. Pharmacol. 12:683575. doi: 10.3389/fphar.2021.683575
Received: 21 March 2021; Accepted: 30 April 2021;
Published: 13 May 2021.
Edited by:
Elena Gazzano, University of Turin, ItalyReviewed by:
Lana Nezic, University of Banja Luka, Bosnia and HerzegovinaTeodora Alexa-Stratulat, Grigore T. Popa University of Medicine and Pharmacy, Romania
Copyright © 2021 Kim, Ku, Hong, Kim, Kwon, Park, Jung, Shin and Ko. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seong-Gyu Ko, epiko@khu.ac.kr
 Min Jeong Kim1
Min Jeong Kim1 Hyo In Kim
Hyo In Kim Seong-Gyu Ko
Seong-Gyu Ko