Beneficial Effects of Bioactive Compounds in Mulberry Fruits against Cisplatin-Induced Nephrotoxicity
Abstract
:1. Introduction
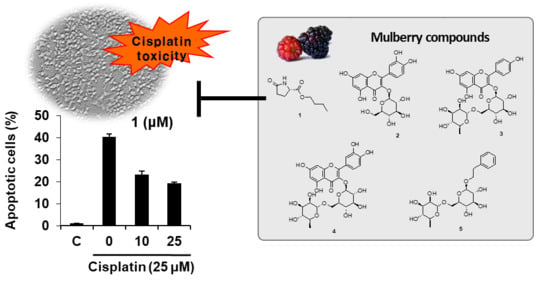
2. Results and Discussion
2.1. Isolation and Structural Identification of Compounds
2.2. Bioactivities
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Cell Culture
3.5. Renoprotective Effect against Cisplatin-Induced Cytotoxicity in LLC-PK1 Kidney Cells
3.6. Western Blot Analysis
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ma, P.; Zhang, S.; Su, X.; Qiu, G.; Wu, Z. Protective effects of icariin on cisplatin-induced acute renal injury in mice. Am. J. Transl. Res. 2015, 7, 2105–2114. [Google Scholar] [PubMed]
- Peres, L.A.B.; Cunha Júnior, A.D. Acute nephrotoxicity of cisplatin: Molecular mechanisms. J. Bras. Nefrol. 2013, 35, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wang, J. Renal drug transporters and their significance in drug—Drug interactions. Acta Pharm. Sin. B 2016, 6, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Havasi, A.; Borkan, S.C. Apoptosis and acute kidney injury. Kidney Int. 2011, 80, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Panichpisal, K.; Kurtzman, N.; Nugent, K. Cisplatin nephrotoxicity: A review. Am. J. Med. Sci. 2007, 334, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Mohrmann, M.; Ansorge, S.; Schmich, U.; Schönfeld, B.; Brandis, M. Toxicity of ifosfamide, cyclophosphamide and their metabolites in renal tubular cells in culture. Pediatr. Nephrol. 1994, 8, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Servais, H.; Ortiz, A.; Devuyst, O.; Denamur, S.; Tulkens, P.M.; Mingeot-Leclercq, M.P. Renal cell apoptosis induced by nephrotoxic drugs: Cellular and molecular mechanisms and potential approaches to modulation. Apoptosis 2008, 13, 11–32. [Google Scholar] [CrossRef] [PubMed]
- Ries, F.; Klastersky, J. Nephrotoxicity induced by cancer chemotherapy with special emphasis on cisplatin toxicity. Am. J. Kidney Dis. 1986, 8, 368–379. [Google Scholar] [CrossRef]
- Kintzel, P.E. Anticancer drug—Induced kidney disorders. Drug Saf. 2001, 24, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Leekha, A.; Gurjar, B.S.; Tyagi, A.; Rizvi, M.A.; Verma, A.K. Vitamin C in synergism with cisplatin induces cell death in cervical cancer cells through altered redox cycling and p53 upregulation. J. Cancer Res. Clin. Oncol. 2016, 142, 2503–2514. [Google Scholar] [CrossRef] [PubMed]
- Zhen, S.; Lu, J.J.; Wang, L.J.; Sun, X.M.; Zhang, J.Q.; Li, X.; Luo, W.J.; Zhao, L. In vitro and in vivo synergistic therapeutic effect of cisplatin with human papillomavirus16 E6/E7 CRISPR/Cas9 on cervical cancer cell line. Transl. Oncol. 2016, 9, 498–504. [Google Scholar] [CrossRef] [PubMed]
- McKim, A.; Walter, A.; Sheely, K.; Manahan, K.; Geisler, J. An economic analysis of cisplatin alone versus cisplatin doublets in the treatment of women with advanced or recurrent cervical cancer. Eur. J. Gynaecol. Oncol. 2016, 37, 353–356. [Google Scholar] [PubMed]
- Wang, S.; Xie, J.; Li, J.; Liu, F.; Wu, X.; Wang, Z. Cisplatin suppresses the growth and proliferation of breast and cervical cancer cell lines by inhibiting integrin β5-mediated glycolysis. Am. J. Cancer Res. 2016, 6, 1108. [Google Scholar] [PubMed]
- Han, M.S.; Han, I.H.; Lee, D.; An, J.M.; Kim, S.N.; Shin, M.S.; Yamabe, N.; Hwang, G.S.; Yoo, H.H.; Choi, S.J. Beneficial effects of fermented black ginseng and its ginsenoside 20 (S)-Rg3 against cisplatin-induced nephrotoxicity in LLC-PK1 cells. J. Ginseng Res. 2016, 40, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Min, W.K.; Park, M.H.; Lee, J.K.; Jin, H.K.; Bae, J.S. Neuropeptide Y protects kidney against cisplatin-induced nephrotoxicity by regulating p53-dependent apoptosis pathway. BMB Rep. 2016, 49, 288. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.P.; Ou, T.T.; Wang, C.J. Mulberry (sang shen zi) and its bioactive compounds, the chemoprevention effects and molecular mechanisms in vitro and in vivo. J. Tradit. Complement. Med. 2013, 3, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.R.; Lee, J.S.; Lee, K.R.; Kim, Y.E.; Baek, N.I.; Hong, E.K. Effects of mulberry ethanol extracts on hydrogen peroxide-induced oxidative stress in pancreatic β-cells. Int. J. Mol. Med. 2014, 33, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Salih, N.D.; Hazir, N.S.M.; Hamid, M.H.A. The effect of mulberry (Morus sp.) tea supplement on acetaminophen induced renal failure in rats. Lab. Sci. 2015, 1, 1. [Google Scholar]
- Peanparkdee, M.; Iwamoto, S.; Borompichaichartkul, C.; Duangmal, K.; Yamauchi, R. Microencapsulation of bioactive compounds from mulberry (Morus alba L.) leaf extracts by protein–polysaccharide Interactions. Int. J. Food Sci. Technol. 2016, 51, 649–655. [Google Scholar] [CrossRef]
- Grajek, K.; Wawro, A.; Kokocha, D. Bioactivity of Morus alba L. extracts—An overview. Int. J. Pharm. Sci. Res. 2015, 6, 3110. [Google Scholar]
- Memon, A.A.; Memon, N.; Luthria, D.L.; Bhanger, M.I.; Pitafi, A.A. Phenolic acids profiling and antioxidant potential of mulberry (Morus laevigata W., Morus nigra L., Morus alba L.) leaves and fruits grown in Pakistan. Pol. J. Food Nutr. Sci. 2010, 60, 25–32. [Google Scholar]
- Andallu, B.; Suryakantham, V.; Lakshmi Srikanthi, B.; Reddy, G.K. Effect of mulberry (Morus indica L.) therapy on plasma and erythrocyte membrane lipids in patients with type 2 diabetes. Clin. Chim. Acta 2001, 314, 47–53. [Google Scholar] [CrossRef]
- Lee, S.R.; Park, J.Y.; Yu, J.S.; Lee, S.O.; Ryu, J.Y.; Choi, S.Z.; Kang, K.S.; Yamabe, N.; Kim, K.H. Odisolane, a novel oxolane derivative, and antiangiogenic constituents from the fruits of mulberry (Morus alba L.). J. Agric. Food Chem. 2016, 64, 3804–3809. [Google Scholar] [CrossRef] [PubMed]
- Yuping, T.; Biao, Y.; Jie, H.; Tao, W.; Yongzheng, H. The chemical constituents from the bulbs of Ornithogalum caudatum. J. Chin. Pharm. Sci. 2001, 10, 169–171. [Google Scholar]
- Han, J.T.; Bang, M.H.; Chun, O.K.; Kim, D.O.; Lee, C.Y.; Baek, N.I. Flavonol glycosides from the aerial parts of Aceriphyllum rossii and their antioxidant activities. Arch. Pharm. Res. 2004, 27, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.M.; Lapsley, K.; Jeong, W.S.; Lachance, P.A.; Ho, C.T.; Rosen, R.T. Antioxidative phenolic compounds isolated from almond skins (Prunus amygdalus batsch). J. Agric. Food Chem. 2002, 50, 2459–2463. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Li, J.; Wang, N.L.; Yao, X.S. Flavonoids and a new polyacetylene from Bidens parviflora Willd. Molecules 2008, 13, 1931–1941. [Google Scholar] [CrossRef] [PubMed]
- Baumes, R.; Bayonove, C.; M’Bairaroua, O.; Tapiero, C. Synthesis and NMR spectral properties of grape monoterpenyl glycosides. Carbohydr. Res. 1990, 207, 39–56. [Google Scholar]
- Florea, A.-M.; Büsselberg, D. Cisplatin as an anti-tumor drug: Cellular mechanisms of activity, drug resistance and induced side effects. Cancers 2011, 3, 1351–1371. [Google Scholar] [CrossRef] [PubMed]
- Rajeswaran, A.; Trojan, A.; Burnand, B.; Giannelli, M. Efficacy and side effects of cisplatin-and carboplatin-based doublet chemotherapeutic regimens versus non-platinum-based doublet chemotherapeutic regimens as first line treatment of metastatic non-small cell lung carcinoma: A systematic review of randomized controlled trials. Lung Cancer 2008, 59, 1–11. [Google Scholar] [PubMed]
- Lee, H.L.; Kang, K.S. Protective effect of ginsenoside Rh3 against anticancer drug-induced apoptosis in LLC-PK1 kidney cells. J. Ginseng Res. 2017, 41, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Garcia, M.E.; Quiroga, A.G.; Castro, J.; Ortiz, A.; Aller, P.; Mata, F. Inhibition of p38-MAPK potentiates cisplatin-induced apoptosis via GSH depletion and increases intracellular drug accumulation in growth-arrested kidney tubular epithelial cells. Toxicol. Sci. 2009, 111, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kim, K.H.; Moon, S.W.; Lee, H.; Kang, K.S.; Lee, J.W. Synthesis and biological evaluation of chalcone analogues as protective agents against cisplatin-induced cytotoxicity in kidney cells. Bioorg. Med. Chem. Lett. 2015, 25, 1929–1932. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Kim, Y.J.; Han, I.H.; Lee, D.; Ham, J.; Kang, K.S.; Lee, J.W. The synthesis of sulforaphane analogues and their protection effect against cisplatin induced cytotoxicity in kidney cells. Bioorg. Med. Chem. Lett. 2015, 25, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.R.; Lee, D.; Eom, H.J.; Lee, S.R.; Lee, K.R.; Kang, K.S.; Kim, K.H. Identification and mechanism of action of renoprotective constituents from peat moss Sphagnum palustre in cisplatin-induced nephrotoxicity. J. Funct. Foods 2016, 20, 358–368. [Google Scholar] [CrossRef]
- Abdelrahman, A.M.; Al Salam, S.; AlMahruqi, A.S.; Mansour, M.A.; Ali, B.H. N-acetylcysteine improves renal hemodynamics in rats with cisplatin-induced nephrotoxicity. J. Appl. Toxicol. 2010, 30, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Zhang, Z.; Cohen, D.M. MAPK signaling and the kidney. Am. J. Physiol. Ren. Physiol. 2000, 279, F593–F604. [Google Scholar] [CrossRef] [PubMed]
- Omori, S.; Hida, M.; Ishikura, K.; Kuramochi, S.; Awazu, M. Expression of mitogen-activated protein kinase family in rat renal development. Kidney Int. 2000, 58, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Johnson, T.S.; Thomas, G.L.; Watson, P.F.; Wagner, B.; Nahas, A.M. Apoptosis and caspase-3 in experimental anti-glomerular basement membrane nephritis. J. Am. Soc. Nephrol. 2001, 12, 485–495. [Google Scholar] [PubMed]
- Jeon, J.H.; Kim, D.K.; Shin, Y.; Kim, H.Y.; Song, B.; Lee, E.Y.; Kim, J.K.; You, H.J.; Cheong, H.; Shin, D.H. Migration and invasion of drug-resistant lung adenocarcinoma cells are dependent on mitochondrial activity. Exp. Mol. Med. 2016, 48, e277. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Yu, J.S.; Lee, S.R.; Hwang, G.S.; Kang, K.S.; Park, J.G.; Kim, H.Y.; Kim, K.H.; Yamabe, N. Beneficial Effects of Bioactive Compounds in Mulberry Fruits against Cisplatin-Induced Nephrotoxicity. Int. J. Mol. Sci. 2018, 19, 1117. https://doi.org/10.3390/ijms19041117
Lee D, Yu JS, Lee SR, Hwang GS, Kang KS, Park JG, Kim HY, Kim KH, Yamabe N. Beneficial Effects of Bioactive Compounds in Mulberry Fruits against Cisplatin-Induced Nephrotoxicity. International Journal of Molecular Sciences. 2018; 19(4):1117. https://doi.org/10.3390/ijms19041117
Chicago/Turabian StyleLee, Dahae, Jae Sik Yu, Seoung Rak Lee, Gwi Seo Hwang, Ki Sung Kang, Jae Gyu Park, Hyun Young Kim, Ki Hyun Kim, and Noriko Yamabe. 2018. "Beneficial Effects of Bioactive Compounds in Mulberry Fruits against Cisplatin-Induced Nephrotoxicity" International Journal of Molecular Sciences 19, no. 4: 1117. https://doi.org/10.3390/ijms19041117