A New Surface Charge Neutralizing Nano-Adjuvant to Potentiate Polymyxins in Killing Mcr-1 Mediated Drug-Resistant Escherichia coli
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis of Ni Doped ZnO Nanoparticles (NZO)
2.2. BP Nanosheets and Ni Doped ZnO-BP (NZB) Nanocomposite Syntheses
2.2.1. BP Nanosheet Synthesis
2.2.2. NZB Nanocomposite Syntheses
2.3. Preparation of the Mcr-1 Expression Plasmid
2.4. Characterization
2.4.1. Material Properties
2.4.2. Zeta Potential Measurement
2.4.3. Bacterial Strains, Growth, and Plasmids
2.4.4. Expression Analysis of Mcr-1 Protein
2.4.5. Preparation of Bacterial Cells
2.4.6. Evaluation of Antibacterial Activity
2.4.7. Morphological Characterization of Bacteria
2.4.8. Reactive Oxygen Species (ROS) Production Determination
2.4.9. Biocompatibility Assays and Morphological Change
3. Results and Discussion
3.1. Material Properties
3.1.1. Phase Composition
3.1.2. XPS Results
3.1.3. Morphology and Microstructure
3.2. Antibacterial Activity
3.2.1. Evaluation of Antibacterial Activity of NZB against E. coli Strains
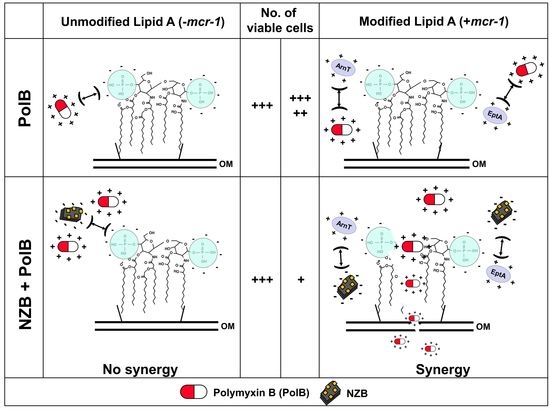
3.2.2. Specific Synergistic Action of NZB to PolB on Mcr-1 Expressing E. coli Strains
3.3. Morphological Characterization of Bacteria
3.4. Plausible Mechanism of NZB: Neutralizing Charge of Bacterial Surface
3.5. Reactive Oxygen Species (ROS) Production
3.6. Biocompatibility of NZB
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mehrad, B.; Clark, N.M.G.; Zhanel, G.; Lynch, J.P. Antimicrobial resistance in hospital acquired gram-negative bacterial infections. Chest 2015, 147, 1413–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, D.; Jeannot, K.; Xiao, Y.; Knapp, C.W. Editorial: Horizontal gene transfer mediated bacterial antibiotic resistance. Front. Microbiol. 2019, 10, 1933. [Google Scholar] [CrossRef] [Green Version]
- Vaara, M. Polymyxins and their potential next generation as therapeutic antibiotics. Front. Microbiol. 2019, 10, 1689. [Google Scholar] [CrossRef]
- He, X.; Deng, Z.; Xu, W.; Li, Y.; Xu, C.; Chen, H.; Shen, J. A novel dual-response chemosensor for bioimaging of Exogenous/Endogenous hypochlorite and hydrazine in living cells, Pseudomonas aeruginosa and zebrafish. Sens. Actuators B 2020, 321, 128450. [Google Scholar] [CrossRef]
- He, X.; Chen, H.; Xu, C.; Fan, J.; Xu, W.; Li, Y.; Deng, H.; Shen, J. Ratiometric and colorimetric fluorescent probe for hypochlorite monitor and application for bioimaging in living cells, bacteria and zebrafish. J. Hazard. Mater. 2020, 388, 122029. [Google Scholar] [CrossRef]
- He, X.; Ding, F.; Sun, X.; Zheng, Y.; Xu, W.; Ye, L.; Chen, H.; Shen, J. Renovated multifunctional colorimetric/fluorometric sensor for simultaneous detection, imaging of pH variance and antimicrobial therapies. Sens. Actuators B 2021, 332, 129496. [Google Scholar] [CrossRef]
- Zhang, H.; Srinivas, S.; Xu, Y.; Wei, W.; Feng, Y. Genetic and biochemical mechanisms for bacterial Lipid A modifiers associated with polymyxin resistance. Trends Biochem. Sci. 2019, 44, 973–988. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Falagas, M.E.; Kasiakou, S.K. Colistin: The revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin. Infect. Dis. 2005, 40, 1333–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paterson, D.L.; Harris, P.N. Colistin resistance: A major breach in our last line of defense. Lancet Infect. Dis. 2015, 16, 132–133. [Google Scholar] [CrossRef]
- Ghirga, F.; Stefanelli, R.; Cavinato, L.; Sciuto, A.L.; Corradi, S.; Quaglio, D.; Calcaterra, A.; Casciaro, B.; Loffredo, M.R.; Cappiello, F.; et al. A novel colistin adjuvant identified by virtual screening for ArnT inhibitors. J. Antimicrob. Chemother. 2020, 75, 2564–2572. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Horinouchi, T.; Furusawa, C. Prediction of antibiotic resistance by gene expression profiles. Nat. Commun. 2014, 5, 5792. [Google Scholar] [CrossRef] [Green Version]
- Naskar, A.; Kim, K.-s. Nanomaterials as delivery vehicles and components of new strategies to combat bacterial infections: Advantages and limitations. Microorganisms 2019, 7, 356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alomary, M.N.; Ansari, M.A. Proanthocyanins-capped biogenic TiO2 nanoparticles with enhanced penetration, antibacterial and ROS mediated inhibition of bacteria proliferation and biofilm formation: A comparative approach. Chemistry 2021. [Google Scholar] [CrossRef]
- Ansari, M.A.; Asiri, S.M.M. Green synthesis, antimicrobial, antibiofilm and antitumor activities of superparamagnetic γ-Fe2O3 NPs and their molecular docking study with cell wall mannoproteins and peptidoglycan. Int. J. Biol. Macromol. 2021, 171, 44–58. [Google Scholar] [CrossRef]
- Naskar, A.; Lee, S.; Lee, Y.; Kim, S.; Kim, K.-s. A new nano-platform of erythromycin combined with Ag nano-particle ZnO nano-structure against methicillin-resistant Staphylococcus aureus. Pharmaceutics 2020, 12, 841. [Google Scholar] [CrossRef] [PubMed]
- Naskar, A.; Lee, S.; Kim, K.-s. Antibacterial potential of Ni-doped zinc oxide nanostructure: Comparatively more effective against Gram-negative bacteria including multidrug resistant strains. RSC Adv. 2020, 10, 1232–1242. [Google Scholar] [CrossRef] [Green Version]
- Food and Drug Administration (FDA). Select Committee on GRAS Substances (SCOGS) Opinion: Zinc Salts; FDA: Washington, DC, USA, 2015. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=182.8991 (accessed on 5 December 2020).
- Naskar, A.; Lee, S.; Kim, K.-S. Easy one-pot low-temperature synthesized Ag-ZnO nanoparticles and their activity against clinical isolates of methicillin-resistant Staphylococcus aureus. Front. Bioeng. Biotechnol. 2020, 8, 216. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Nilghaz, A.; Lin, Y.; Xu, J.; Lu, X. Black phosphorus and its biomedical applications. Theranostics 2018, 8, 1005–1026. [Google Scholar] [CrossRef]
- Naskar, A.; Kim, K.-s. Black phosphorus nanomaterials as multi-potent and emerging platforms against bacterial infections. Microb. Pathog. 2019, 137, 103800. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gao, L.-F.; Li, L.; Hu, C.-X.; Yang, Q.-Q.; Zhu, Z.-Y.; Peng, R.; Wang, Q.; Peng, Y.; Jin, J.; et al. Negatively charged 2D black phosphorus for highly efficient covalent functionalization. Mater. Chem. Front. 2018, 2, 1700–1706. [Google Scholar] [CrossRef]
- Naskar, A.; Kim, S.; Kim, K.-s. A nontoxic biocompatible nanocomposite comprising black phosphorus with Au–ɣ-Fe2O3 nanoparticles. RSC Adv. 2020, 10, 16162–16167. [Google Scholar] [CrossRef]
- Salman, M.; Rizwana, R.; Khan, H.; Munir, I.; Hamayun, M.; Iqbal, A.; Rehman, A.; Amin, K.; Ahmed, G.; Khan, M.; et al. Synergistic effect of silver nanoparticles and polymyxin B against biofilm produced by Pseudomonas aeruginosa isolates of pus samples in vitro. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2465–2472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmerman, S.M.; Lafontaine, A.-A.J.; Herrera, C.M.; Mclean, A.B.; Tren, M.S. A whole-cell screen identifies small bioactives that synergize with polymyxin and exhibit antimicrobial activities against multidrug-resistant bacteria. Antimicrob. Agents Chemother. 2020, 64, e01677-19. [Google Scholar] [CrossRef]
- Ouyang, J.; Liu, R.-Y.; Chen, W.; Liu, Z.; Xu, Q.; Zeng, K.; Deng, L.; Shen, L.; Liu, Y.-N. A black phosphorus based synergistic antibacterial platform against drug resistant bacteria. J. Mater. Chem. B 2018, 6, 6302–6310. [Google Scholar] [CrossRef] [PubMed]
- Lincopan, N.; Santana, M.R.A.; Faquim-Mauro, E.; da Costa, M.H.B.; Carmona-Ribeiro, A.M. Silica-based cationic bilayers as immunoadjuvants. BMC Biotechnol. 2009, 9, 5. [Google Scholar] [CrossRef] [Green Version]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef] [Green Version]
- Baba, T.; Ara, T.; Hasegawa, M.; Takai, Y.; Okumura, Y.; Baba, M.; Datsenko, K.A.; Tomita, M.; Wanner, B.L.; Mori, H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol. Syst. Biol. 2006, 2, 2006. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.-s.; Kim, T.; Pan, J.-G. In vitro evaluation of ciclopirox as an adjuvant for polymyxin B against gram-negative bacteria. J. Antibiot. 2015, 68, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Albetran, H.M.; Alheshibri, M.H.; Timoumi, A.; Algarou, N.A.; Akhtar, S.; Slimani, Y.; Almessiere, M.A.; Alahmari, F.S.; Baykal, A.; et al. Synthesis of electrospun TiO2 nanofibers and characterization of their antibacterial and antibiofilm potential against Gram-positive and Gram-negative bacteria. Antibiotics 2020, 9, 572. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wang, J.; Lin, X.; He, J.; Liu, H.; Lei, Y.; Chu, Z. Black phosphorus/TiO2 composite photoanode with enhanced photoelectrical performance. ChemElectroChem 2017, 4, 2373–2377. [Google Scholar] [CrossRef]
- Vijayaprasath, G.; Murugan, R.; Palanisamy, S.; Prabhu, N.M.; Mahalingam, T.; Hayakawa, Y.; Ravi, G. Role of nickel doping on structural, optical, magnetic properties and antibacterial activity of ZnO nanoparticles. Mater. Res. Bull. 2016, 76, 48–61. [Google Scholar] [CrossRef]
- Simar, S.; Sibley, D.; Ashcraft, D.; Pankey, G. Colistin and polymyxin B minimal inhibitory concentrations determined by etest found unreliable for gram-negative Bacilli. Ochsner J. 2017, 17, 239–242. [Google Scholar]
- Kim, J.; Hwang, B.K.; Choi, H.; Wang, Y.; Choi, S.H.; Ryu, S.; Jeon, B. Characterization of mcr-1-harboring plasmids from pan drug-resistant Escherichia coli strains isolated from retail raw chicken in South Korea. Microorganisms 2019, 7, 344. [Google Scholar] [CrossRef] [Green Version]
- Prasad, K.S.; Prasad, S.K.; Ansari, M.A.; Alzohairy, M.A.; Alomary, M.N.; AlYahya, S.; Srinivasa, C.; Murali, M.; Ankegowda, V.M.; Shivamallu, C. Tumoricidal and bactericidal properties of ZnONPs synthesized using Cassia auriculata leaf extract. Biomolecules 2020, 10, 982. [Google Scholar] [CrossRef]
- Guan, R.; Kang, T.; Lu, F.; Zhang, Z.; Shen, H.; Liu, M. Cytotoxicity, oxidative stress, and genotoxicity in human hepatocyte and embryonic kidney cells exposed to ZnO nanoparticles. Nanoscale Res. Lett. 2012, 7, 602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reshma, V.G.; Mohanan, P.V. Cellular interactions of zinc oxide nanoparticles with human embryonic kidney (HEK 293) cells. Colloids Surf. B Biointerfaces 2017, 157, 182–190. [Google Scholar]






| Strain | Presence of mcr-1 2 | Monotherapy | Synergistic Combination | |||||
|---|---|---|---|---|---|---|---|---|
| MIC (μg·mL−1) | MIC (μg·mL−1) | Maximum FICINZB/PolB | ||||||
| PolB | NZB | PolB | Fold Change to Monotherapy | NZB | Fold Change to Monotherapy | |||
| E. colistrains | ||||||||
| BW25113 | − | 0.8 | 250 | 0.8 | 1 | 250 | 1 | 2 |
| Keio-arnT | − | 0.8 | 250 | 0.2 | 4 | 31.25 | 8 | 0.375 |
| Keio-eptA | − | 0.8 | 250 | 0.2 | 4 | 31.25 | 8 | 0.375 |
| NCCP16283 | + | 3.2 | 250 | 0.8 | 4 | 12.5 | 20 | 0.3 |
| NCCP16284 | + | 3.2 | 250 | 0.8 | 4 | 12.5 | 20 | 0.3 |
| BAA-2471 | − | 0.8 | 250 | 0.4 | 2 | 125 | 2 | 1 |
| BAA-2340 | − | 0.8 | 250 | 0.4 | 2 | 125 | 2 | 1 |
| KS7000 3 | − | 0.8 | 250 | 0.4 | 2 | 125 | 2 | 1 |
| KS8000 4 | + | 6.4 | 250 | 1.6 | 4 | 6.25 | 40 | 0.275 |
| Non-E. coli Gram-negative strains | ||||||||
| NCCP16285 | + | 6.4 | 250 | 6.4 | 1 | >250 | >1 | >2 |
| ATCC19606 | − | 3.2 | >250 | 3.2 | 1 | >250 | 1 | 2 |
| ATCC27853 | − | 3.2 | >250 | 3.2 | 1 | >250 | 1 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, H.; Naskar, A.; Lee, S.; Kim, S.; Kim, K.-S. A New Surface Charge Neutralizing Nano-Adjuvant to Potentiate Polymyxins in Killing Mcr-1 Mediated Drug-Resistant Escherichia coli. Pharmaceutics 2021, 13, 250. https://doi.org/10.3390/pharmaceutics13020250
Cho H, Naskar A, Lee S, Kim S, Kim K-S. A New Surface Charge Neutralizing Nano-Adjuvant to Potentiate Polymyxins in Killing Mcr-1 Mediated Drug-Resistant Escherichia coli. Pharmaceutics. 2021; 13(2):250. https://doi.org/10.3390/pharmaceutics13020250
Chicago/Turabian StyleCho, Hyejin, Atanu Naskar, Sohee Lee, Semi Kim, and Kwang-Sun Kim. 2021. "A New Surface Charge Neutralizing Nano-Adjuvant to Potentiate Polymyxins in Killing Mcr-1 Mediated Drug-Resistant Escherichia coli" Pharmaceutics 13, no. 2: 250. https://doi.org/10.3390/pharmaceutics13020250